Introduction
Previous clinical trials have reported that adolescent and young adult (AYA) patients treated for T-cell acute lymphoblastic leukemia (T-ALL) have lower disease-free survival (DFS) and overall survival (OS) and increased toxicity as compared to younger patients. Results for the AYA cohort treated on two COG international phase 3 trials for T-ALL are reported here.
Patients and Methods
AALL0434 enrolled 1562 T-ALL patients (2007-2014) and AALL1231 enrolled 615 T-ALL patients (2014-2019) treated on augmented BFM (ABFM) regimens. AYA patients were defined as age ≥16 years of age (AALL0434: n=226; AALL1231: n = 107). Both studies allowed enrollment up to age 31 years.
On AALL0434, participants were randomized to receive escalating dose methotrexate (CMTX) without leucovorin rescue + pegaspargase or high dose MTX (HDMTX) + leucovorin rescue. Intermediate and high-risk patients were randomized to receive or not receive six 5-day courses of nelarabine (Nel). Risk group assignments in AYA vs non-AYA patients can be found in Table 1. On AALL1231, participants were randomized to receive or not receive bortezomib in Induction and Delayed Intensification.
Key differences between AALL0434 and AALL1231 ABFM backbones included the use of prednisone in induction in AALL0434 versus dexamethasone in AALL1231, and cranial radiation therapy in the majority of AALL0434 participants versus only in patients with central nervous system involvement in AALL1231.
Results
On AALL0434, the 4-year DFS for AYA was 81% compared to 84% for those <16 years old (P=0.5965), and OS was 88% and 90% respectively (P=0.3478). AYA patients randomized to CMTX (n=70) had a superior DFS compared to those randomized to HDMTX (n=72) with 4-year DFS 96% versus 78% respectively (P=0.0038). No disadvantage was seen among AYA subjects who received nelarabine versus those who did not, as shown by a 4-year DFS 85% versus 89%, respectively (N=54) (P=0.8116), albeit the sample size was small. On AALL1231, the 4-year EFS for AYA (n=107) was 82.7±3.9% compared to 82.2 ±1.7% for non-AYA (n=508) (P=0.727), and OS was 86.5 ±3.5% and 88.4 ±1.5% respectively (P=0.553). No difference in outcome was seen for AYA patients randomized to receive/not receive bortezomib.
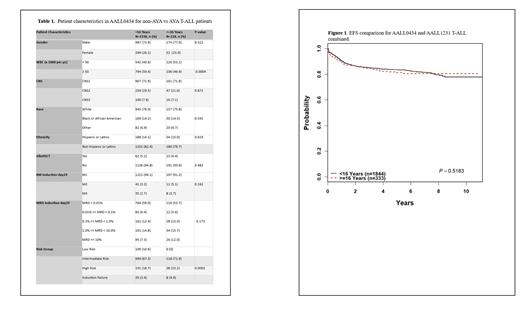
Combined outcome data for AALL0434 and AAL1231 demonstrates 4-year EFS for AYA (n=333) of 82.2+2.4% vs 83.9+0.9% for non-AYA (n=1844) (P=0.52) (Figure 1) and OS of 87.5+2.1% vs 90+0.8% respectively (P=0.19).
Significant differences in patient characteristics in AALL0434 were higher initial white blood counts (P=0.0004) and higher risk group category (P=0.0002) for AYA patients. There were no significant differences in the AALL0434 AYA cohort compared to younger patients in CNS disease status, race, ethnicity, remission status at end of induction, or day 29 end of induction minimal residual disease (MRD).
Toxicity rates were not significantly different in non-AYA vs AYA patients with the exception of upper respiratory infection (9.4% vs 1.8%; P=0.0001) and osteonecrosis (4.8% vs 17.7%; P<0.0001) in AALL0434. Rates of sepsis, catheter-related infection, and other infections were not significantly different between AYA and younger patients in AALL0434.
Analysis of patient characteristics and toxicity rates in AALL1231, as well as analysis of the combined toxicity data for AALL0434 and AAL1231, are currently underway and will be presented at the meeting.
Conclusion
COG AALL0434 and AALL1231 show outstanding overall outcomes for AYA patients with no significant difference in survival between AYA and non-AYA patients on either study, despite differences in the ABFM backbones and the higher risk features seen among the AYA group in AALL0434. There were similar occurrences of therapy-related toxicities for AYA and non-AYA study participants on AALL0434. Despite small numbers, CMTX holds a survival advantage for AYA T-ALL patients, with no survival disadvantage seen among AYA T-ALL patients who received Nel. Bortezomib does not offer significant benefit for AYA T-ALL patients.
Disclosures
Wood:Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Institutional Laboratory Services Agreement; Kite: Other: Institutional Laboratory Services Agreement; Novartis: Other: Institutional Laboratory Services Agreement; Beckman-Coulter: Honoraria; Becton-Dickinson: Honoraria; Beam: Other: Institutional Laboratory Services Agreement; Wugen: Other: Institutional Laboratory Services Agreement; Macrogenics: Other: Institutional Laboratory Services Agreement; Biosight: Other: Institutional Laboratory Services Agreement. Zweidler-McKay:Immunogen Inc.: Current Employment. Raetz:Pfizer: Research Funding; Bristol Myer Squibb: Other: DSMC. Teachey:Neoimmune Tech: Research Funding; BEAM: Research Funding; Sobi: Membership on an entity's Board of Directors or advisory committees, Research Funding; Jazz: Membership on an entity's Board of Directors or advisory committees, Research Funding.